Acids And Bases
The Arrhenius Theory of Acids and Bases. 1-3 is a Strong Acid 4-7 is a Weak Acid 7 is Neutral 8-10 is a Weak Alkali 11-14 is a Strong Alkali.

Acids Bases And Ph Good Science
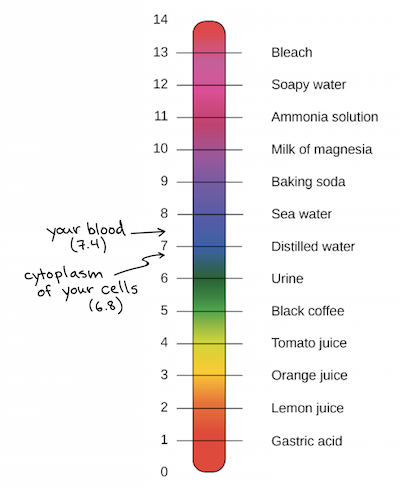
Ph Scale Acids Bases Ph And Buffers Article Khan Academy
Acids And Bases For The Mcat Everything You Need To Know Shemmassian Academic Consulting
Acids bases and pH.
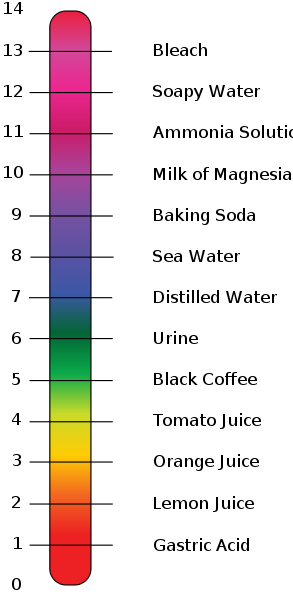
Acids and bases. C10 acids bases and salts 1. Vinegar is diluted acetic acid which is. A base is a substance that renders hydroxyl ionOH in their aqueous.
So HNO 3 will be nitric acid. They play an efficient role inside or outside of our body. There is a simple set of rules for these acids.
Our mothers never store pickles in metal containers. Acids An acid is a substance that releases H ions in an aqueous solution Aqueous means water Example. The acid-base theory of Brønsted has been used thoroughly in the history of acid and base.
Baking soda is the most common base used in cooking. Acids Bases and Salts Class 10 Summary Explanation and Notes of Acids Bases and Salts Science Chapter 2. Ionization Of Acids And Bases.
Some common weak acids and bases are given here. Nomenclature of common acids. Lewis acidbase motif is one of the most applicable theories and it extends the denotation of acids and bases beyond H and OH- ions.
To identify acids from bases and the relative strength of each chemists tend to use a pH scale. This is the currently selected item. Both Acids and bases make the critical part and parcel of our livelihood.
Acids Bases and Salts. If it has a lot of hydrogen ions then it is an acid. Examples of bases and alkalis in everyday life.
Johannes Nicolaus Brønsted - Thomas Martin Lowry Acids and Bases The Brønsted or Brønsted-Lowry theory describes acid-base reactions as an acid releasing a proton and a base accepting a proton. Acids Bases and Salts 154 SCIENCE AND TECHNOLOGY Notes MODULE - 2 Matter in our Surroundings 8 ACIDS BASES AND SALTS From generations our parents have been using tamarind or lemon juice to give shiny look to the copper vessels. Both acids and bases are important aspects when we study chemistry.
Whether a liquid is an acid or base depends on the type of ions in it. Everything in the world is an acid a base or neutral. Weak acid-base equilibria Opens a modal Conjugate acidbase pairs Opens a modal Relationship between Ka and Kb Opens a modal Relationship between Ka and Kb Opens a modal Ka and acid strength Opens a.
Common salt and sugar has often been used as an effective. Lewis acids and bases are commonly classified according to their hardness or softness. Acids and bases interact with each other in what is called a neutralization reactionThe products of the reaction are a salt and water.
One of the earliest tests to determine acids from bases was the litmus test. This activity will teach students about how to tell whether an object is an acid a base or neutral. He explains how increases in the hydronium ion or hydrogen ion concentration can lower the pH and crea.
Acids and Bases 2. From the formation of the food to the decomposition of any substance acids and bases play a crucial role in our everyday life. Weak acids and bases are only partially ionized in their solutions whereas strong acids and bases are completely ionized when dissolved in water.
Acids and bases 1. PH pOH and the pH scale. A chemical patch turned red for acids blue for bases.
Water autoionization and Kw. The pH is neutralized to 7. Examples of acids in everyday life.
A base is a molecule or ion able to accept a hydrogen ion from an acid. Almost all liquids are either acids or bases to some degree. Bases Alkalis Vocabulary Quiz Bibliography pH review.
Paul Andersen explains pH as the power of hydrogen. In 1884 the Swedish chemist Svante Arrhenius proposed two specific classifications of compounds. Acids and Bases.
Arrhenius acids and bases. Bases play an essential role in baking for their ability to react with acids and release carbon dioxide gas. When dissolved in an aqueous solution certain ions were released into the solution.
Anything with a pH above 7 is basic. An acid is any hydrogen-containing substance that is capable of donating a proton hydrogen ion to another substance. The term strong in the name refers to the acids ability to release hydrogen H molecules which allows it to become ionized when placed into a solution of waterWeak acids do not have this ability.
Mineral acids are acids prepared from minerals. LEARNING OUTCOMES Define acid and acid anhydride Investigate the reactions of non-oxidising acids with metals carbonates hydrogen carbonates and bases Define base and alkali Investigate the reaction of bases with ammonium salts Relate acidity and alkalinity to the pH scale Discuss the strength of acids and alkalis on the basis of their completeness of. Acids bases and pH.
Acids and Bases are encountered daily in chemistry and our everyday life. Also Check Dilute Acids. Acids Bases and Salts Notes of CBSE Class 10 Science Chapter with detailed explanation of the chapter Acids bases and salts along with meanings of difficult words.
The conjugate base is the ion or molecule remaining after the acid has lost its proton and the conjugate acid is the species created when the base accepts the proton. More complex acids have oxygen in the compound. Difference Between Acid and Base.
BrønstedLowry acids and bases. An Arrhenius acid is a compound that increases the concentration of H ions that are present when added. In this context hard implies small and nonpolarizable and soft indicates larger atoms that are more polarizable.
When hydrochloric acid is dissolved in water the compound separates into chlorine ions Cl- and hydrogen ions H. There are several definitions of what constitutes an acid. Brønsted-Lowry acids and bases.
Gas bubbles form in batter or dough when baking soda combines with any acidic ingredients such as yogurt lemon juice or buttermilk. By the 1884 definition of Svante Arrhenius Sweden an acid is a material that can release a proton or hydrogen ion H. A Detailed Analysis of Lewis Acids Bases.
There are seven strong acid. The most common characteristic of bases is their bitter taste and soapy feel. This chart provides the nomenclature of some common anions and acids.
While the acid definition is pretty much the same as that proposed by Arrhenius a hydrogen ion is a proton the definition of what constitutes a base is much broader. For example Hydrochloric acid HCl Sulphuric Acid H 2 SO 4 and nitric acid HNO 3 etc. List of Strong Acids.
Given here is the complete explanation of the chapter along with examples and all the exercises Question and. A strong acid is one that dissolves in water. Strong acids are not named as such because they are more powerful than other acids.
Furthermore weak acids and bases are very common and we encounter them often both in the academic problems and in everyday life. Acids and bases are two special kinds of chemicals. Any polyatomic ion with the suffix -ate uses the suffix -ic as an acid.
The formation of conjugate acids and bases is central to the Brønsted-Lowry definition of acids and bases. Anything with a pH below 7 is acidic. Acids and Bases Definition.
H alkalialkaline earth metal cations boranes Zn 2 typical soft acids. Arrhenius acids and bases.
Acids And Bases
Uses And Properties Of Acids And Bases All You Need To Know About Acids And Bases

Acids Bases And Living Things Lesson Helpteaching Com
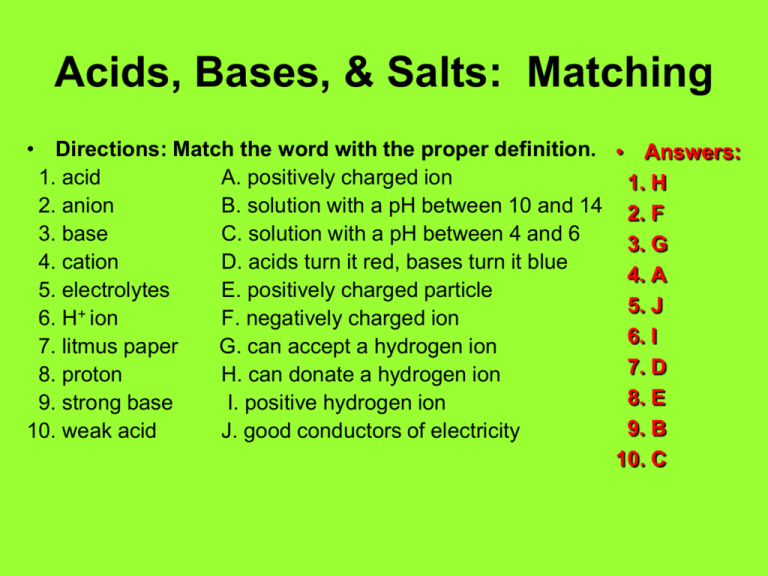
Acids Bases Salts Matching

Acids And Bases Boundless Chemistry

Kids Science Acids And Bases
Acids And Bases Reaction With Each Other Don T Memorise Youtube

The Characteristic Properties Of Acids Bases Cie Igcse Chemistry Revision Notes
Comments
Post a Comment