Hydrogen Gas Formula
There are three isotopes of hydrogen. Explosive reactions occur upon ignition of mixtures of nitrogen trifluoride with good reducing agents such as ammonia hydrogen hydrogen sulfide or methane.
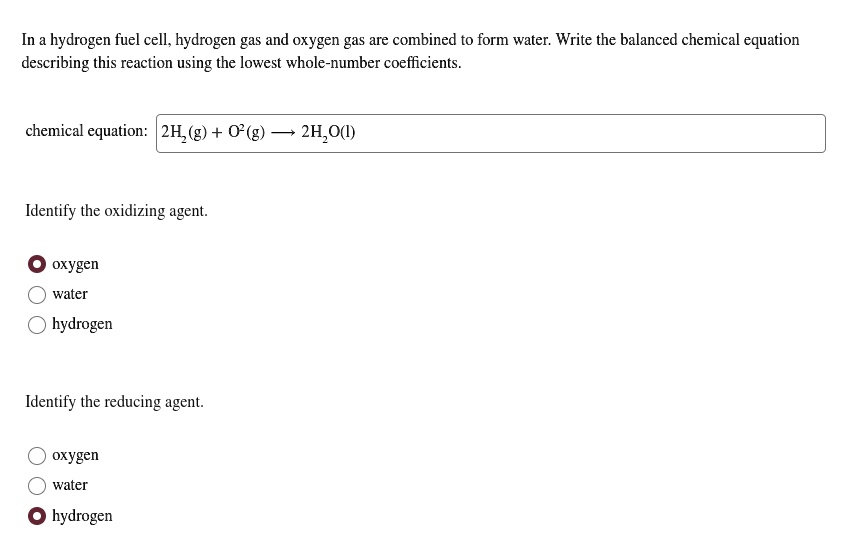
Solved Hydrogen Fuel Cell Hydrogen Gas And Oxygen Gas Are Combined To Form Water Write The Balanced Chemical Equation Describing This Reaction Using The Lowest Whole Number Coefficients Chemical Equation 2h G 2h O Identify
Molecules And Ions

Laboratory Preparation Of Hydrogen Gas Explanation With Illustration
Hydrogen bonding occurs between a hydrogen atom and an electronegative atom eg oxygen fluorine chlorine.
Hydrogen gas formula. Normal hydrogen at room temp contains 25 of para form 75 ortho form. All you need to do to create this nano bubble cloud of molecular hydrogen gas is drop one of our H 2 Molecular Hydrogen tablets into a 16-oz. Weight - Mass vs.
Heavy water is also possible in which one or more of the atoms of hydrogen consist of deuterium symbol D or tritium symbol T. The formula of the gas constant from the ideal gas law equation is. Hydrogen is also prevalent on Earth in the form of chemical compounds such as hydrocarbons and water.
The Hydrogen Convergence Alliance H2Korea is a public-private consultative body established by the Korean government in 2017 to promote. Gas Facts includes charts and tables and interactive conversion formulas related to the chemical and physical properties of our cryogenic liquid and compressed gas products as well as an online tool for estimating the cost of using nitrogen oxygen or argon. In an energy ecosystem that relies on H 2 as a fuel for power generation large volumes of H 2 will have to be generated.
Hydrogen gas forms the simplest covalent bond in the diatomic hydrogen molecule. HydrogenNatural Gas Mixtures. A stream of hydrogen from a leak is almost invisible in daylight.
The usual chemical formula for water assumes the hydrogen atoms consist of the isotope protium one proton no neutrons. The bond is weaker than an ionic bond or a covalent bond but stronger than van der Waals forces 5 to 30 kJmol. It is the ratio of the product of pressure and volume to the product of mole and temperature.
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen and an oxygen mixture gas 67 hydrogen mixed with 33 oxygen produced from deionized water by electrolysis with a hydrogenoxygen generator model AMS-H-03. Nitrogen gas was also applied and adjusted the oxygen concentration to 21.
In the year 1885 on the basis of experimental observations Balmer proposed the formula for correlating the wave number of the spectral lines emitted and the energy shells involved. The nitrogen and oxygen which makes up the bulk of the atmosphere also exhibits covalent bonding in forming diatomic molecules. Glass of room-temperature filtered water.
At standard temperature and pressure hydrogen is a nontoxic nonmetallic odorless tasteless colorless and highly combustible diatomic gas with the molecular formula H 2. Hydrogen as a vehicle fuel can be stored either as a high-pressure gas or as a cryogenic liquid Section 22. Hydrogen fluoride is a chemical compound with the chemical formula H FThis colorless gas or liquid is the principal industrial source of fluorine often as an aqueous solution called hydrofluoric acidIt is an important feedstock in the preparation of many important compounds including pharmaceuticals and polymers eg.
122 Odor Color and Taste Pure hydrogen is odorless colorless and tasteless. Weight - the Gravity Force. Mixtures of hydrogen carbon monoxide or methane and oxygen difluoride are exploded when a spark is discharged Mellor 2 Supp.
The Gas Constant R In PV nRT. Hydrogen is the lightest element. Where hydrogen from electrolysis has to compete on a heating value basis with natural gas ie where the hydrogen sales price benchmark is set by the price of natural gas such as in the case of admixture to the natural gas grid or when used as feedstock in industry such as in refineries it is not cost-competitive under the current energy and CO 2 price regimes and existing regulation.
A solution of the gas in water is called hydrochloric acid. The lifting power of 1 cubic foot of hydrogen gas is about 007 lb at C 760 mm pressure. Consideration is being given to an entire economy based on solar- and nuclear-generated hydrogen.
Learn the Uses of Hydrogen Atomic Mass of Hydrogen Atomic Number and Its Properties. The ideal gas constant is the proportionality constant in the ideal gas equation. Its chemical formula is HCl.
Other forms of the chemical formula for water include D 2 O DHO T 2 O and THO. Methane - Thermophysical Properties - Chemical Physical and Thermal Properties of. The halogens such as chlorine also exist as diatomic gases by forming covalent bonds.
Hydrogen gas under ordinary conditions is a mix of 2 kinds of molecules known as ortho- para-hydrogen which differ from one another by the spins of their electrons nuclei. A hydrogen bond is classified as a type of weak chemical bond. P1 is the initial absolute pressure and V1 is the initial volume the work of adiabatic compression is given by a formula of the following form.
This formula is given as. At standard conditions hydrogen is a gas of diatomic molecules having the formula H 2It is colorless odorless non-toxic and highly combustibleHydrogen is the most abundant chemical substance in the universe constituting roughly 75 of all normal matter. Hydrogen - Thermophysical Properties - Chemical Physical and Thermal Properties of Hydrogen - H 2.
Hydrogen is a highly combustible diatomic gas with the molecular formula H2. This series of the hydrogen emission spectrum is. Formula of Gas Constant.
Hydrogen - Specific Heat - Specific heat of Hydrogen Gas - H 2 - at temperatures ranging 175 - 6000 K. Asclepius Meditec Co Ltd Shanghai China was directly introduced into the inhalation chamber at a rate of 3 Lmin. At the right temperature you create molecular hydrogen water in about 90 seconds at a truly biologically meaningful concentration of up to 8 ppm of HH 2.
PV nRT where P is pressure V is volume T is temperature and n is the number of molecules of gas youre keeping track of. This gas constant referred to as a physical constant that is introduced in different fundamental equations in the physical sciences such as the ideal gas law. Hydrogen gas and liquid unit conversion tables - weight gas volume liquid volume pounds kilograms standard cubic feet standard cubic meters gallons liters.
Learn more about hydrogen chloride including its properties. The formula for understanding this is. Hydrogen chloride a compound of the elements hydrogen and chlorine a gas at room temperature and pressure.
The gas constant R is also known as the universal molar or ideal gas constant. Thus gas turbines operating on hydrogen could provide the needed grid firming while at the same time generating significantly less carbon dioxide CO 2 emissions. The hydrogen fuel cell is a developing technology that will allow great amounts of electrical power to be obtained using a source of hydrogen gas.
Dihydrogen H2 Chemspider
Properties Of Hydrogen Introduction To Chemistry
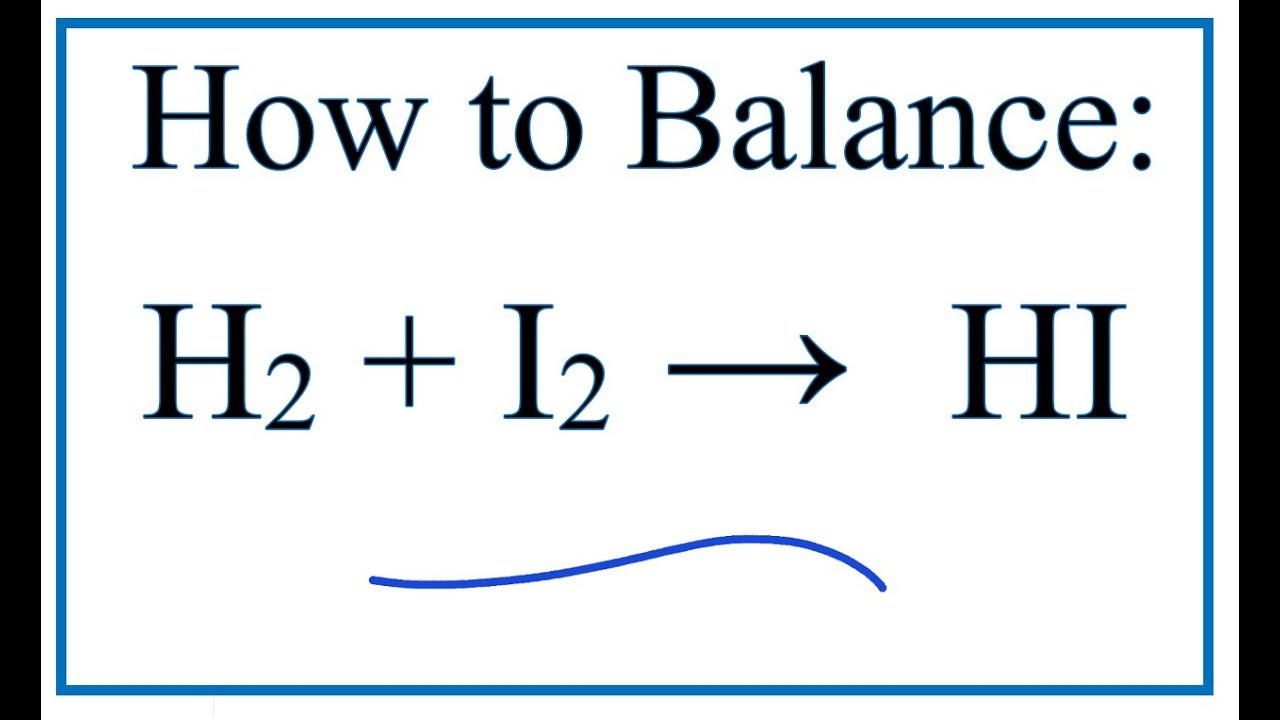
How To Balance H2 I2 Hi Hydrogen Gas Iodine Gas Youtube
Hydrogen Energies And Spectrum

Fields That Could Be Achieved With Nitrogen And Hydrogen Gas At 100 Atm Download Scientific Diagram
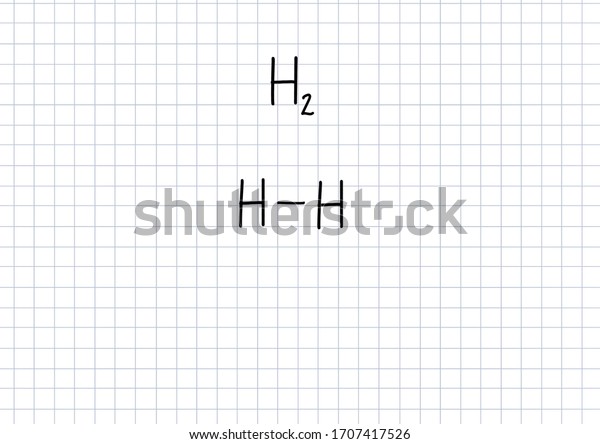
Hydrogen Gas Formula Handwritten Chemical Formula Stock Illustration 1707417526

Pod Write The Chemical Equations For The Following 1 Hydrogen Gas Reacts With Oxygen Gas To Form Water 2 Nitrogen Gas Mixes With Hydrogen Gas To Form Ammonia Ppt Download

Hydrogen Facts Hydrogen Element Facts

Comments
Post a Comment