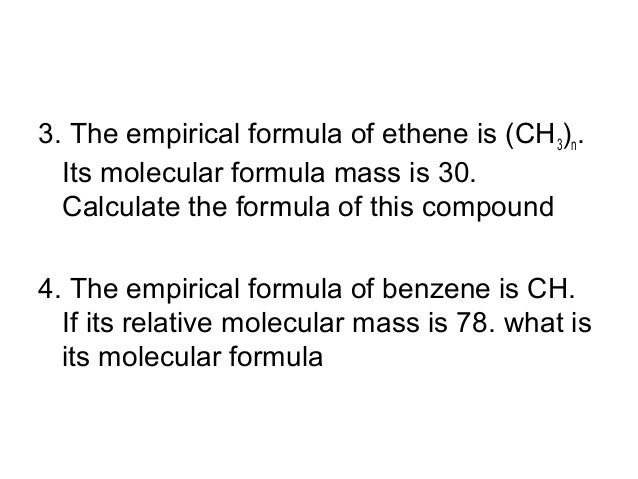
Empirical Formula Of Ethene
Particle where the particle is found in an atom relative mass relative charge orbiting the nucleus 1 1840 1 in the nucleus 3 b How many electrons neutrons and protons are there in the ion shown. The formula for ethyl alcohol or ethanol is C 2 H 5 OH or CH 3 CH 2 OH.

How To Name Alkenes General Empirical Structural Skeletal Displayed Formula Naming Branched Substituted Alkenes Molecular Structure Nomenclature Isomers Molecule Shapes Bond Angles Ks5 As A2 A Level Organic Chemistry Revision Notes Doc
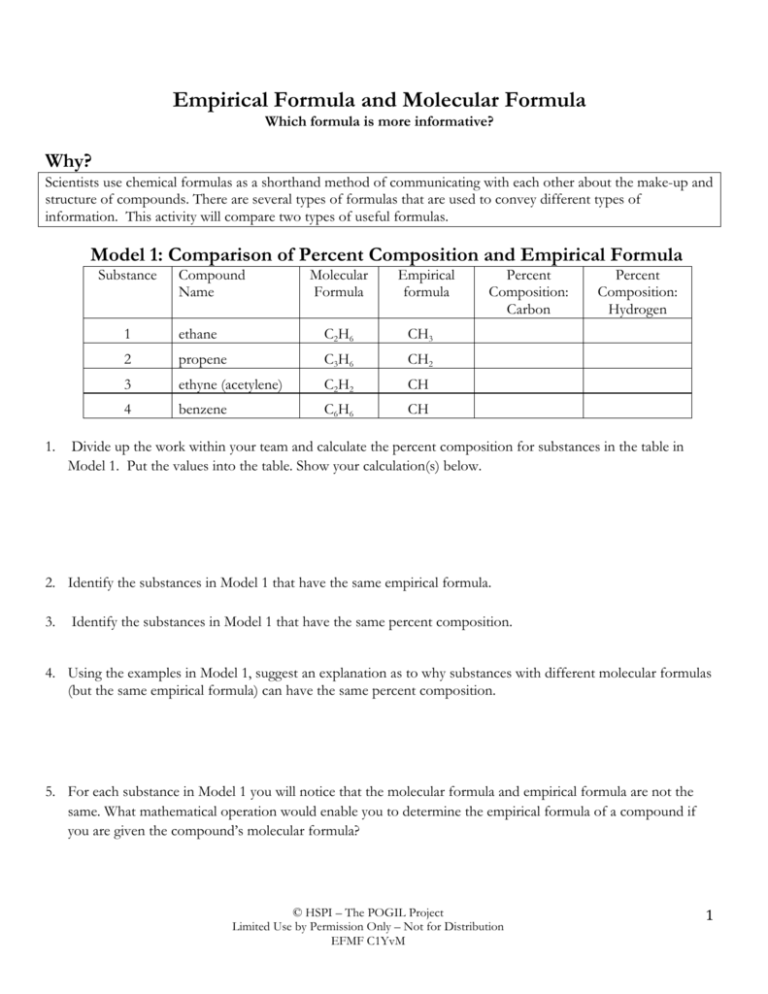
Empirical Formula And Molecular Formula
G E C H C2 S1 Q4 Elevise
Empirical Formula Hill Notation.
Empirical formula of ethene. Fatty acids made up of ten or more carbon atoms are nearly insoluble in water and because. AUS-e-TUTE is a science education website providing notes quizzes tests exams games drills worksheets and syllabus study guides for high school science students and teachers. Empirical Formula to Molecular Formula.
Br C F F F Draw a dot-and-cross diagram to show all the outer electrons in this molecule. The empirical formula represents the simplest whole number ratio of various atoms present in a compound. A Complete the table.
I Both A and R are true and R is the correct explanation of A. 44Ca 20 2 number of electrons. Iv Both A and R are false.
Atomic and molecular masses mole concept and molar mass percentage composition empirical and molecular formula chemical reactions stoichiometry and calculations based on stoichiometry. As the May 13 2013 c Sodium chloride solution can be made from dilute hydrochloric acid and sodium hydroxide solution. An empirical formula is simply the smallest whole number ratio between atoms in a compound.
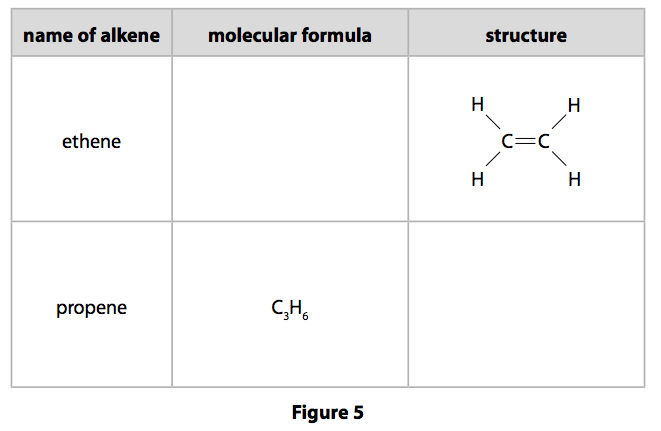
The second member of the series is propene propylene. Alkane alkene alkyne series and their gradation in properties and the relationship with the molecular mass or molecular formula. Once the empirical formula is estimated we can also find the molecular formula if the molar mass is known.
The empirical formula represents the simplest whole number ratio of various atoms present in a compound. The empirical formula represents the simplest whole number ratio of various atoms present in a compound. I Both A and R are true and R is the correct explanation of A.
Iii A is false but R is true. Iii A is false but R is true. Discovery of Electron Proton and Neutron atomic number isotopes and isobars.
The empirical formula for benzene was long known but its highly polyunsaturated structure with just one hydrogen atom for each carbon atom was challenging to determine. Percentage composition empirical and molecular formula chemical reactions stoichiometry and calculations based on stoichiometry. Now the empirical formula is made by placing each of the whole numbers as the subscript to respective elements.
What is the empirical formula of the compound of silver and carbon. Asked Mar 5 2018 in Class XI Chemistry by nikita74 Expert 112k points. Ethene C 2 H 4 is the simplest alkene.
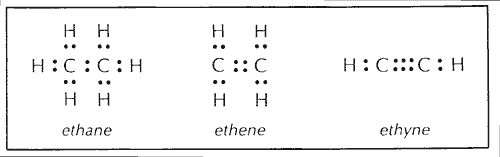
Page 1 of 9. C C H H H H H. The empirical mass of ethene is half of its molecular mass.
Carboxylic acids and salts having alkyl chains longer than eight carbons exhibit unusual behavior in water due to the presence of both hydrophilic CO 2 and hydrophobic alkyl regions in the same moleculeSuch molecules are termed amphiphilic Gk. Structural formula must be given for. Five years later Archibald Scott Couper published the structural formula of ethanol.
Shows the simplest whole number ratio of atoms of each element in the compound A2 Organic naming Basic definitions to know Displayed formula. Ii A is true but R is false. Page 1 of 9.
Amphi both or amphipathic. Determine the ratio of the molar mass to the empirical formula mass. Shows all the covalent bonds present in a molecule Functional group is an atom or group of atoms which when present in different molecules causes them to have similar chemical properties.
Ii A is true but R is false. For the preparation of dilute volumetric solutions or for direct use cHCl 5 moll 5 N Combi-Titrisol. Bohrs model and its limitations concept of shells and subshells dual nature of matter and light de Broglies relationship Heisenberg.
The simplest alkene is ethene with a formula of C2H4. If a precipitate will form enter its empirical formula in the last column. The empirical formula for ethene is CH2 the smallest whole number ratio between the atoms.
The empirical mass of ethene is half of its molecular mass. Assertion A. Iv Both A and R are false.
Read no So leadII iodide is insoluble and will form a precipitate PbI 2 s. C 23 H 38 N 7 O 17 P 3 S xLi CAS No. Isomerism structural chain position iii Homologous series characteristics with examples.
Archibald Scott Couper in 1858 and Johann Josef Loschmidt in 1861 31 suggested possible structures that contained multiple double bonds or multiple rings but too little evidence was then available to help chemists decide. C empirical formula D general formula Total for Question 12 1 mark P60789A0724 7. A Show by calculation that the empirical formula of this compound is CBrF 3 2 b The diagram shows the displayed formula of a molecule of Halon 1301.
Thomsons model and its limitations. However the addition of a strong acid serves to catalyze the addition of water and in this way alcohols may be prepared from alkenes. 80957 free acid basis Compare Product No.
A molecular formula is the exact number of each type of atom necessary to build a specific molecule. 505 x 10-3 mol of C means 505 x 10-3 mol of X in 0152 g RMM of X 0152 g 505 x 10-3 g mol-1 3010 g mol-1 The molecular formula is also CH 2O and X is actually methanal or. The molecular formula could be any multiple of the empirical formula eg.
2 c The boiling point of Halon 1301 is 58 C. Explain why Halon 1301 has a low boiling. One mole of compound contains the Avogadro number of the molecule or formula unit 602214076 1023 moles.
C 2H 4O 2 or C 3H 6O 3 since these would all have the same percentage mass ratios. Each carbon atom in ethene commonly called ethylene has a trigonal planar structure. The empirical mass of ethene is half of its molecular mass.
The empirical mass of ethene is half of its molecular mass. Product Number Product Description SDS. A mole is a counting unit used to determine the number of molecules atoms ions or molecular formula units in a certain compound.
Ethanol is a compound of carbon hydrogen and oxygen elements was described by Antoine Lavoisier and its chemical formula was determined by Nicolas-Theodore de Saussure in 1808. It is similar to other counting units such as a pair 2 and a dozen 12. Alkanes alkenes alkynes u p to 5 carbon atoms.
For example if sulfuric acid is dissolved in water it is completely ionized to the hydronium ion H 3 O and this strongly acidic pK a -174 species effects hydration of ethene and other alkenes. Draw the skeletal formula of 223-trimethylbutane. When naming an alkene one has to take care to note which carbons have the double bonds.
Ammonium nitrate reacts with calcium hydroxide. For example C2H4 is the molecular formula for ethene. 2 CLE 2019 06204319 1 Atoms contain particles called electrons neutrons and protons.
16 Ethene reacts with hydrogen in the presence of a heated nickel catalyst to form ethane.
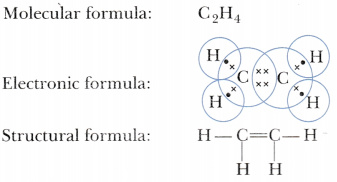
Write Molecular Electronic And Structural Formulae Of Ethene Cbse Class 10 Science Learn Cbse Forum

Ethene 1 2 Dione Formula C2o2 Over 100 Million Chemical Compounds Mol Instincts
Chemistryklipz Files Wordpress Com
Section 6 5 Emperical Versus Molecular Formulas
Official Formula Article About Official Formula By The Free Dictionary
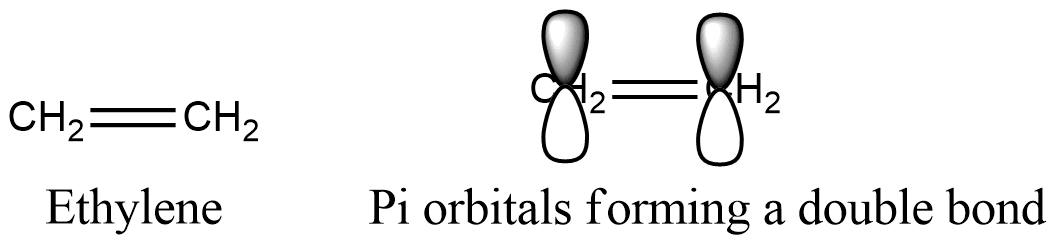
Ethylene Formula
A Introduction Archives Tutormyself Chemistry
Answer All The Question
Comments
Post a Comment